Computing time to aid in studying ‘molecular machines’
By Theresa CarsonMedical Center Public Affairs
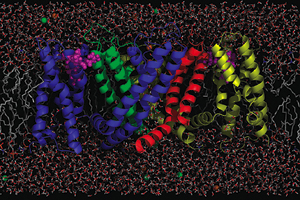 | |
Four million hours of computing time will be granted by the U.S. Department of Energy’s Office of Science to study the voltage gating of a membrane protein upon which human life depends, the potassium channel. The project is overseen by the University’s Institute for Molecular Pediatric Sciences and directed by Benoît Roux, Professor in Pediatrics and Biochemistry & Molecular Biology.
Membrane-associated proteins control the flow of information and material through the cell membrane. These channels govern cell excitability, such as the transmission of nerve impulse and heart muscle-cell contractions. They are complex nanoscale “molecular machines” designed to accomplish specific tasks. Malfunction of one type of channel, sometimes owing to the misbehavior of only a few atoms, can lead to numerous diseases.
The study is designed to help scientists better understand how these channels are able to carry out their functions. “The ultimate goal of this study is to elucidate the mechanism of the voltage-gated potassium channel, a membrane-associated protein functioning as a molecular electric switch,” Roux said.
The first step is to have good models of the structure of the channel in both the closed and open states. To do so, researchers used protein X-ray crystallography to study the position of the atoms in a crystallized state of the potassium channel. In 2005, a team of scientists led by Rod Mackinnon at Rockefeller University succeeded in determining the structure of the open state of the Kv1.2 channel from a rat’s brain. The configuration of the closed state remains unknown, though plausible models can be inferred indirectly from a wide range of experimental data.
“But even with experimentally-determined X-ray structures, static images are not enough,” Roux said. “To understand how these channels really work, one must ultimately be able to ‘visualize’ atom-by-atom how they move and change their shape as a function of time during voltage-gating.”
For example, different parts within the protein called “alpha-helices” are believed to move when the channels open or close in response to the membrane voltage. Until now, picturing these movements has remained elusive. Breaking new ground in understanding how membrane proteins work requires a novel paradigm permitting a tight integration of structural, dynamical and functional data together with theory, modeling and simulations. Roux likened this process to animating a cartoon.
Roux’s work will illuminate the full spectrum of motion to open and close the potassium ion channels. Computer simulations are necessary because it is the only way scientists currently can determine the time-dependent movement of the different parts of the protein as a function of applied membrane voltage.
Roux will process the computations on the Cray XT3 supercomputer, also known as Jaguar, at the Oak Ridge National Laboratory. With 10,400 processing cores and 21 terabytes of memory, Jaguar can perform 54 trillion mathematical calculations per second.
The computer processing hours are made possible through a Department of Energy award named INCITE (Innovative and Novel Computation Impact on Theory and Experiment). To run this project on a single-processor desktop computer would take more than 450 years. Through the INCITE program, processing will take less than a year.
![[Chronicle]](/images/sidebar_header_oct06.gif)