Chemists use model to predict when, where blood will clot
By Steve KoppesNews Office
  | |
University chemists have demonstrated for the first time how to use a simple laboratory model consisting of only a few chemical reactions to predict when and where blood clotting will occur.
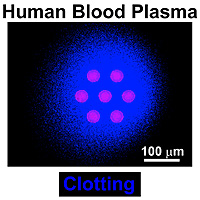
Using microfluidics, the scientists were able to probe blood clotting on surfaces that mimic vascular damage on the micron scale, a unit of measurement much narrower than the diameter of a human hair.
Although scientists understand what occurs during many of the 80 individual chemical reactions involved in blood clotting, many questions remain about the dynamics of the entire reaction network. Rustem Ismagilov, Associate Professor in Chemistry, and graduate students Christian Kastrup, Matthew Runyon and Feng Shen have now developed a technique that will allow them and other scientists to understand the rules governing complex biological reaction networks.
This work has earned Ismagilov the inaugural Journal of Physical Organic Chemistry award for Early Excellence in the Field of Organic Chemistry. Ismagilov’s team presented its latest findings in the Tuesday, Oct. 24 issue of the Proceedings of the National Academy of Sciences.
How finely tuned a human’s blood-clotting system is can mean life or death. “Clotting has to occur at the right place at the right time,” Ismagilov said. “A strong, rapid clotting response is essential to stop bleeding at a wound, but such a clotting response at the wrong spot can block blood vessels and can be life-threatening.” In the past, scientists have typically examined the blood-clotting network using flasks containing homogenous mixtures ยะ the test fluids were the same throughout. But the contents of the circulatory system are not homogeneous, said Kastrup, a Ph.D. student in Chemistry and the PNAS article’s lead author. One of the great virtues of microfluidics technology is its ability to control complex reactions at critical times and locations. “The blood-clotting system contains both fluids and surfaces in an elaborate spatial environment, where localization of chemicals is very important,” he said.
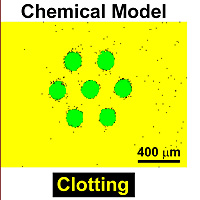
In previous work, the Ismagilov group designed a simple laboratory model to simulate blood clotting. In this model, Ph.D. student Runyon and his associates devised three modules that correspond to the three major stages of clotting: production of chemicals that activate clotting, the inhibition of these activators and formation of the solid clot.
In this model, the scientists used only one chemical reaction in each module instead of the 20-to-30 biochemical reactions that the modules represent. Surprisingly, this simple model adequately reproduced many features of blood clotting.
“There’s a long history in chemistry of using simple models to understand more complex behavior,” Kastrup said. “Instead of looking at hundreds of equations for blood clotting, we reduced it down to three main equations. From these equations we were able to describe a lot of the dynamics of clotting.”
The ability of microfluidics to mimic the flow and geometry of human blood vessels also proved critical.
“We had to use microfluidics to do all of this because that’s how we controlled where everything is,” Ismagilov said of Runyon’s previous work. “It turned out that we got appropriate behavior only if we used geometry similar to those observed in our vascular system. If we changed the geometry to something that didn’t look like a biological system, the chemical system couldn’t function. So geometry and flow were very important.”
In the latest advance, Kastrup used Runyon’s model to see if he could predict when clotting would occur in human blood. The team predicted and verified that clotting occurs only at locations of vascular damage larger than a critical size. “Surprisingly, this simple model made correct, quantitative predictions about blood clotting,” Kastrup said.
Furthermore, the model provided new details about the dynamics of clotting. A big question in blood-clotting studies is the role of a protein called tissue factor. Can tissue factor exist in blood without the presence of clotting?
“From our experiments we see that it’s not the overall concentration of tissue factor that matters, but it’s the localization of it that makes a difference,” Kastrup explained. That means a high concentration of tissue factor at one location will result in clotting, while the same number of molecules spread farther apart will not.
In the future, chemists might be able to apply microfluidics to the study of other complex reaction networks that control various biological functions. And in the medical arena, the technique could become a way to perform rapid and detailed diagnostic tests. “We’d love to see that happen,” Kastrup said.
The National Science Foundation, the Office of Naval Research, the Burroughs Wellcome Fund, the Research Corporation and the Alfred P. Sloan Foundation supported the research.
![[Chronicle]](/images/sidebar_header_oct06.gif)