Researchers studying developmental path for generating B cells from stem cells
By John EastonMedical Center Public Affairs
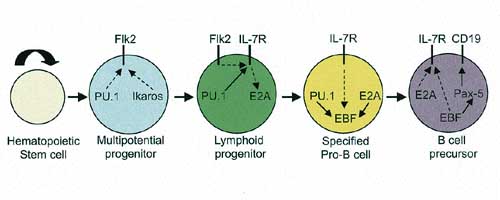 Gene regulatory networks orchestrating the generation of a B-cell precursor from a hematopoietic stem cell are shown above. Four successive, interdependent developmental states are depicted. Each transition is enabled by distinct combinations of regulatory molecules (gene regulatory proteins like PU.1 and signaling receptors like IL-7R). Gene regulators activate or repress target genes whereas signaling receptors induce or modify the activities of gene regulators. | |
Before stem cells of any origin can be used to treat patients, scientists will need to learn how to coax them to develop into the desired cell types. In the Tuesday, Oct. 12 issue of Developmental Cell, University researchers present the first rough road map for this process, suggesting how to lead a hematopoietic stem cell down the narrowing path to becoming an antibody-producing B cell.
The researchers describe four critical stages on the way from a multipotent precursor to a committed B cell and suggest how combinations of regulatory proteins and signaling pathways direct maturing cells through each crossroad, guiding them down one specific developmental path, preparing them to respond to signals yet to come and blocking off other options.
“Our findings reveal considerable complexity, but are promising from the standpoint of directing stem cell differentiation,” said Harinder Singh, the Louis Block Professor in Molecular Genetics & Cell Biology and an Investigator with the Howard Hughes Medical Institute at the University.
“For this one cell type, about which we already knew a great deal, it’s a complicated and elaborate recipe that involves multiple ingredients at each step and their mixing in a particular order. We expect that other cell types will require similarly complex regulatory networks for their generation.
“But the work is also promising,” Singh added. “Once we delineate the components and gain insight into the design principles of such regulatory networks, we may be able to make any kind of cell we want, or even produce hybrids that combine features of different cell types, such as antibody-producing skin cells.”
Singh and colleagues work with hematopoietic stem cells, or HSCs, which give rise to the different types of blood cells. Unlike embryonic stem cells, HSCs already have taken some steps in differentiation and are committed to producing various types of blood cells.
“This is a wonderful model system,” Singh said. “We know more about the differentiation of B cells and red cells than other cell types in the blood system.”
After a series of experiments involving the manipulation of multiple genes encoding regulatory proteins, Singh and colleagues came up with a “hierarchical regulatory network” that orchestrates the differentiation of a stem cell into a committed B-cell precursor.
Five transcriptional regulators guide future B cells along this pathway, activating genes that move the cell to the next stage and enabling the cell to respond to specific chemical signals later on.
“This is a complicated sequence of events,” Singh noted. “There’s no denying it.” At each stage, different markers or receptors appear on the cell surface, which helps the researchers monitor a cell’s progress and enables the cell to reach the next stage.
“To make effective use of stem cells we will have to assemble genetic regulatory networks such as this for each cell type we want to generate,” Singh added.
“This is the next challenge facing the field. Molecular biologists are used to manipulating single genes, but this may require controlling several components in an ordered manner to properly and efficiently direct a stem cell through a given developmental sequence.”
![[Chronicle]](/images/sidebar_header_oct06.gif)